Parvovirus-GLUE: Highlights
These highlights pages aim to provide a brief overview of selected data items contained within the Parvovirus-GLUE project.
EPVs: Dependo.54.Cavia (enRep)
Dependo.54.Cavia (also called enRep) is one of several dependoparvovirus-derived EPVs identified in the germline of guinea pigs (genus Cavia). We identified two groups of elements that spanned the rep gene. The first includes enRep sequences from both guinea pig species examined. The second includes a longer element that spans an entire rep gene. This second group of elements is much older, being shared across rodents species that diverged >70 million years ago.
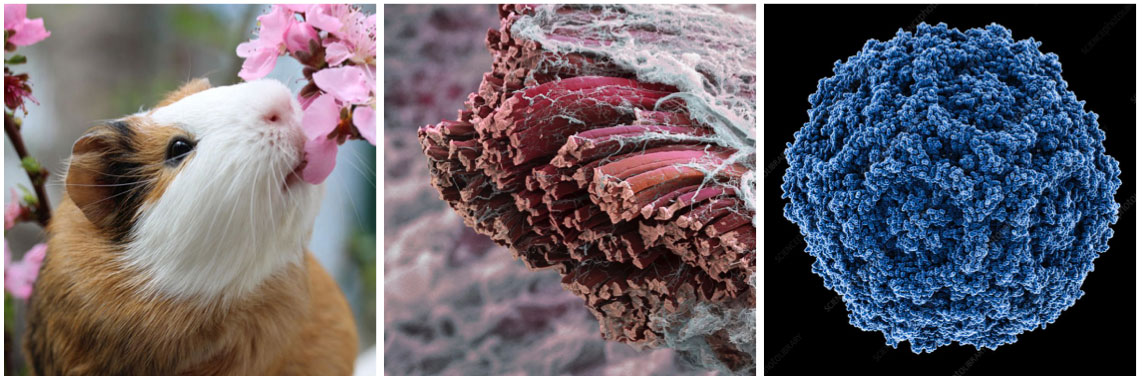
left to right: Guinea pig, Skeletal muscle fibres, parvovirus virion.
While most of this EPV sequence is degraded, the portions included in the enRep-Myo9 gene are intact in multiple species of guinea pig (genus Cavia), consistent with evolution under purifying selection.
The broad expression of enRep-Myo9 mRNA, the conservation of it’s EPV-derived regions in multiple species of guinea pig (genus Cavia), indicate that this host-virus fusion gene encodes a protein with a physiologically relevant role.
The viral portions of enRep-Myo9 derive from an ancient dependoparvovirus (genus Dependoparvovirus) that was incorporated into the genome of caviomorph rodents >6 million years ago.
Related data items:
Viruses: Hamaparvoviruses
Occasionally, our investigations of WGS databases turn up sequences that do not derive from endogenous parvoviral elements (EPVs), but instead from infectious viruses that have contaminated genomic DNA samples. In 2017 we reported sequences derived from viruses belonging to genus Chaphamaparvovirus in WGS data of diverse vertebrate species. Detection and analysis of these sequences indicated that the host range of 'chappaparvoviruses' (as the group was then known) encompassed a diverse range of vertebrate species.
Chaphamaparvoviruses are representatives of a newly described parvovirus subfamily: ''Hamaparvovirinae'. Although relatively little is known about these viruses (most have only been described at sequence-level) it is becoming clear that they are very widely distributed among vertebrate species, and that some are associated with disease. For example, porcine parvovirus 7 (PPV) is one of the organisms associated with Stillbirths Mummification Embryonic Death and Infertility (SMEDI) syndrome in domestic pigs, while mouse kidney parvovirus is associated with “inclusion body nephritis/nephropathy” - a disease of immunocompromised laboratory mice.

Some of the species in which hamaparvoviruses and/or hamaparvovirus-derived EPVs have been identified. Left to right: 'Icthamaparvovirus'-derived EPVs were identified in the tiger-tail seahorse; Porcine parvovirus 7 is an emerging virus of domestic pigs; Mouse kidney parvovirus is associated with nephrotic disease in immunosuppressed laboratory mice; We have identified EPVs derived from unclassified hamaparvovirus-like viruses in a wide range of invertebrate species.
We subsequently performed broader screening in animal genomes and identified EPV sequences derived from unclassified hamaparvovirus-like viruses in arthropods and molluscs, as well as an ichthamaparvovirus-derived EPV identified in the genome of the tigertail seahorse. Ichthamaparvovirus is the second genus defined in subfamily Hamaparvovirinae. Officially it contains only a single species, Syngnathus scovelli chapparvovirus (ScChPV), identified in the gulf pipefish (Syngnathus scovelli). However, phylogenetic evidence supports the inclusion of 'Ichthyic parvovirus' in this genus.
We recently identified icthamaparvovirus-derived EPVs in snakes, providing robust evidence that the host range of this viral genus extends to reptiles. Furthermore, orthologous copies of this EPV were identified in multiple snake species establishing that it integrated into the serpentine germline >50 million years ago. EPV-Icthama.2-Serpentes thus provides the most robust evidence yet that hamaparvoviruses are an ancient lineage and have been associated with vertebrates throughout their evolution.
Related data items:
- Ichthama EPV reference sequence details
- Ichthama ortholog sequences
- Chaphamaparvovirus FASTA from WGS
- Ichthamaparvovirus FASTA from WGS
EPVs: Amdo.1.Ellobius
Amdo.1.Ellobius is an amdoparvovirus-derived EPV, identified in the genome of the Transcaucasian mole vole (Ellobius lutescens) - a species of cricetid rodent inhabiting semi-arid or grassland areas in Central Asia, and notable for its unusual karyotype: only a single sex chromosome is present - with the Y chromosome having been eliminated - and all individuals possess a diploid number of 17 chromosomes. This interesting characteristic has motivated the sequencing of the E.lutescens genome, as well as that of a sister species - the northern mole vole (E. talpinus).
We identified the corresponding empty genomic integration sites in E. talpinus indicating that both elements were incorporated into the E. lutescens germline within the last 10 million years. Intriguingly, in silico pedictions indicated that this replicase could be expressed as a fusion protein with a partial MAFG gene product.

Transcaucasus region - habitat of the Transcaucasian mole vole (Ellobius lutescens).
We identified four further EPV in mammal and reptile genomes that are intermediate between amdoparvoviruses and their sister genus (Protoparvovirus) in terms of their phylogenetic placement and genomic features. In particular, we identify a genome-length EPV in the genome of a pit viper (Protobothrops mucrosquamatus) that is intermediate between proto- and amdoparvoviruses. Notably, it exhibits characteristically amdoparvovirus-like genome features including: (i) a putative middle ORF gene; (ii) a capsid gene that lacks a phospholipase A2 (PLA2) domain; (iii) a genome structure consistent with an amdoparvovirus-like mechanism of capsid gene expression.
More recently, we identify orthologous copies of the Amdoparvovirus-like EPV in additional snake species, establishing it integrated into the genome >100 million years ago. Despite this, some copies encode a replicase gene that appears to have the potential to express intact protein.
Related data items:
Related Publications
Hildebrandt E, Penzes J, Gifford RJ, Agbandje-Mckenna M, and R Kotin
(2020)
Evolution of dependoparvoviruses across geological timescales – implications for design of AAV-based gene therapy vectors.
Virus Evolution
[view]
Pénzes JJ, de Souza WM, Agbandje-Mckenna M, and RJ Gifford
(2019)
An ancient lineage of highly divergent parvoviruses infects both vertebrate and invertebrate hosts.
Viruses
[view]
Callaway HM, Subramanian S, Urbina C, Barnard K, Dick R, Hafentein SL, Gifford RJ, and CR Parrish
(2019)
Examination and reconstruction of three ancient endogenous parvovirus capsid proteins in rodent genomes.
Journal of Virology
[view]
Valencia-Herrera I, Cena-Ahumada E, Faunes F, Ibarra-Karmy R, Gifford RJ*, and G Arriagada*
(2019)
*co-corresponding authors
Molecular properties and evolutionary origins of a parvovirus-derived myosin fusion gene in guinea pigs.
Journal of Virology [view]
Roediger B, Lee Q, Tikoo S, Cobbin JCA, Henderson JM, Jormakka M, O'Rourke MB, Padula MP, Pinello N,
Henry M, Wynne M, Santagostino SF, Brayton CF, Rasmussen L, Lisowski L, Tay SS, Harris DC, Bertram JF,
Dowling JP, Bertolino P, Lai JH, Wu W, Bachovchin WW, Wong JJ, Gorrell MD, Shaban B, Holmes EC, Jolly CJ,
Monette S, Weninger W.
(2018)
An Atypical Parvovirus Drives Chronic Tubulointerstitial Nephropathy and Kidney Fibrosis.
Cell. [view]
Souza WM, Romeiro MF, Fumagalli MJ, Modha S, de Araujo J, Queiroz LH, Durigon EL, Figueiredo LT, Murcia PR, Gifford RJ.
(2017)
Chapparvoviruses occur in at least three vertebrate classes and have a broad biogeographic distribution.
J Gen Virol.
[view]
Pénzes JJ, Marsile-Medun S, Agbandje-McKenna M, and RJ Gifford
(2018)
Endogenous amdoparvovirus-related elements reveal insights into the biology and evolution of vertebrate parvoviruses.
Virus Evolution
[view]